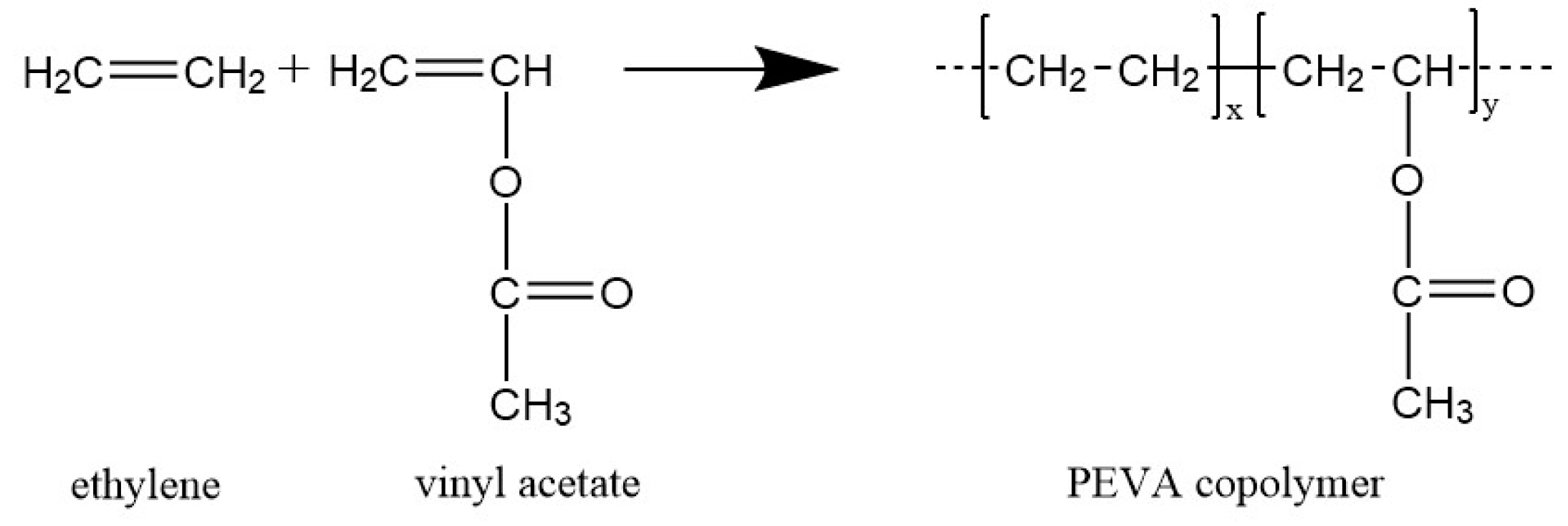
Vinylic Protons Are Acidic Because

The α protons of carbonyl groups are acidic because the negative charge in the enolate can be partially distributed in the oxygen atom.
Vinylic protons are acidic because. The complementary process when a proton is added transferred to a brønsted lowry base is protonation or hydronation. The book indicates the red proton is the more acidic proton. Is this the proper resonance structure for the red proton s conjugate base. Typical proton proton coupling constants.
The species formed is the conjugate acid of that base. The o h and n h protons are exchangeable and this is handy feature because when in doubt you can add a drop of deuterated water d 2 o and make the signal disappear since deuterium does not resonate in the region where protons do. It is not one of the functional groups in the list above but there is a similar proton is table of acids that we learned. The 2 bond coupling between protons on the same alkene carbon referred to as geminal protons is very fine generally 5 hz or lower.
If so then this explains why the red proton is more acidic because it has a delocalized pair of electrons while the blue proton does not have a delocalized pair of electrons. Allyl is a name for this radical. H2s also has a proton attached to an s and has a pka of 7 0 which is fairly acidic. Notice that the proton closest to the carbonyl group is at a higher chemical shift than the proton in cyclohexene 6 05 ppm for cyclohexenone vs.
Deprotonation or dehydronation is the removal transfer of a proton or hydron or hydrogen cation h from a brønsted lowry acid in an acid base reaction the species formed is the conjugate base of that acid. This is not surprising since the proton is not only vinylic but it is also alpha to a carbonyl group. A better question would be what s an allylic position because allylic proton is nothing more than proton hydrogen in allylic position. Other groups that give broad and sometimes deuterium exchangeable signals are the amines amides and thiols.
We know that a proton alpha to a carbonyl group is pulled downfield. Protons c is bonded to an sp2 carbon which makes it a poor acid. The allylic position is the one on carbon next to double bond.