Vinylic Carbon Vs Allylic Carbon
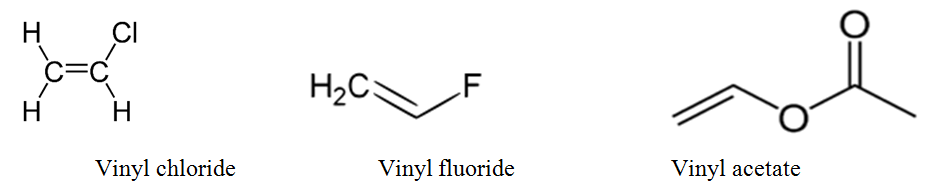
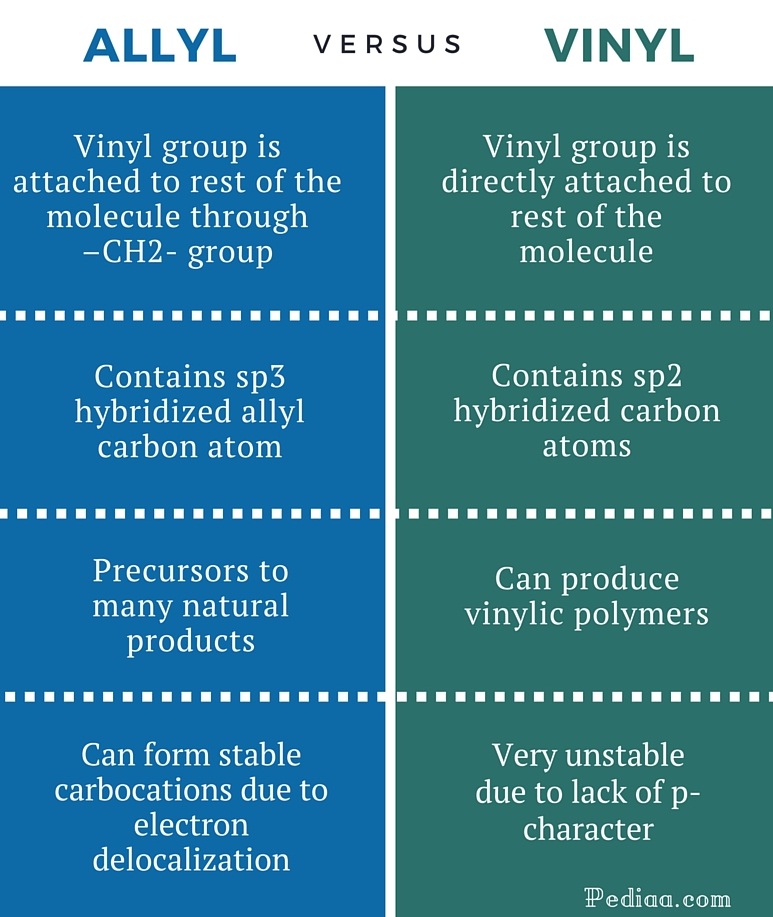
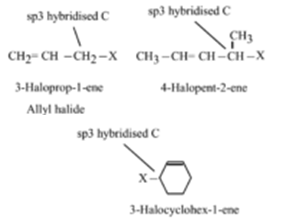
The general formula for vinyl group is r ch ch 2 in which both carbon atoms are bonded with double bond and r is attached at vinylic position.
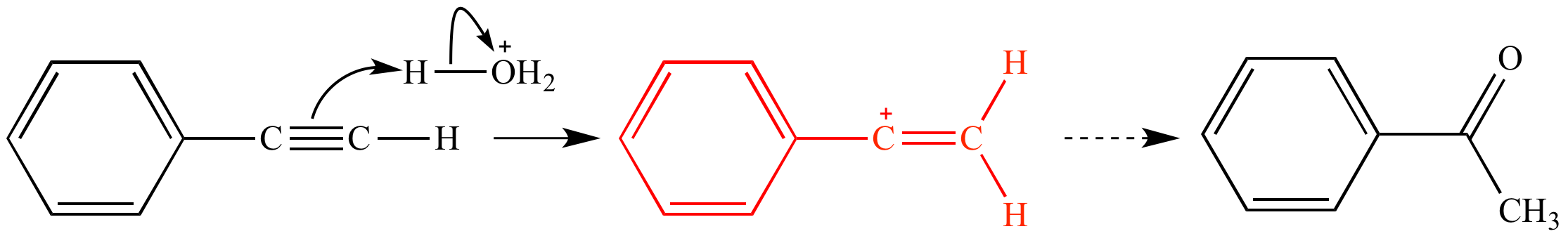
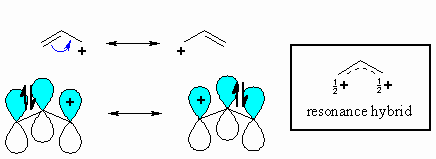
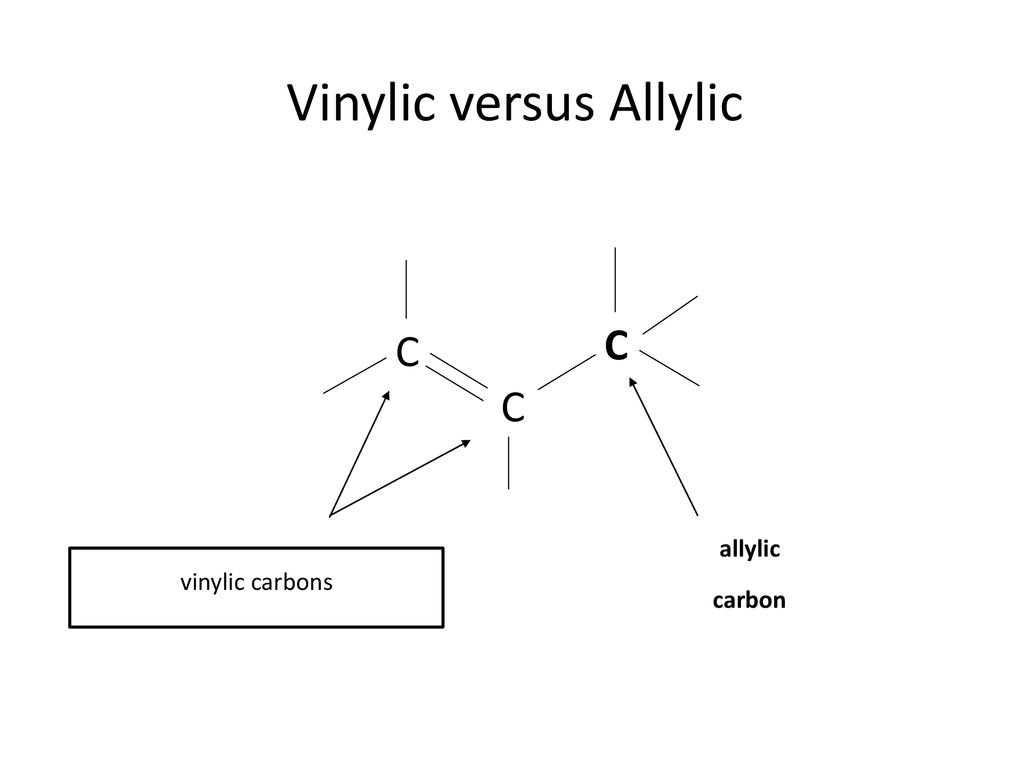
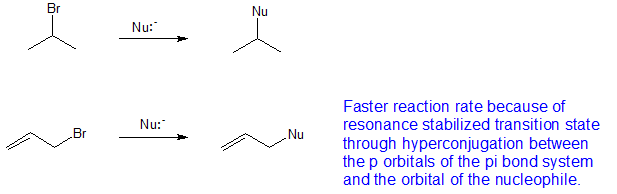
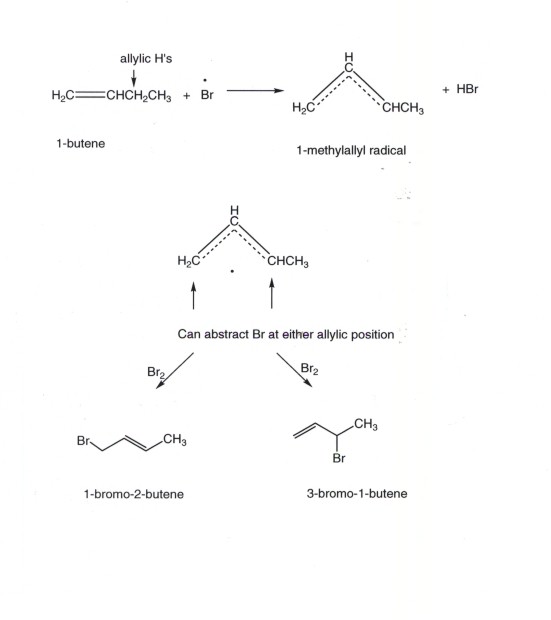
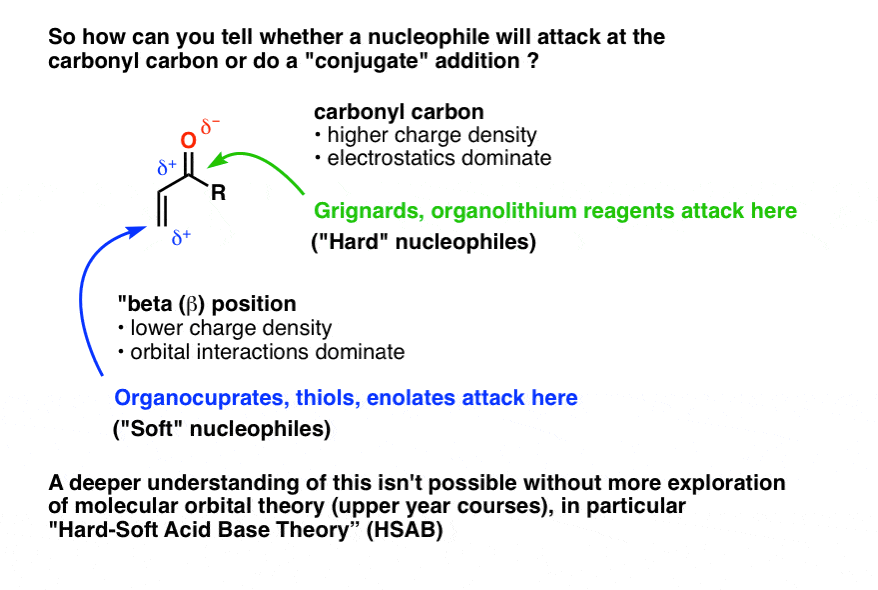
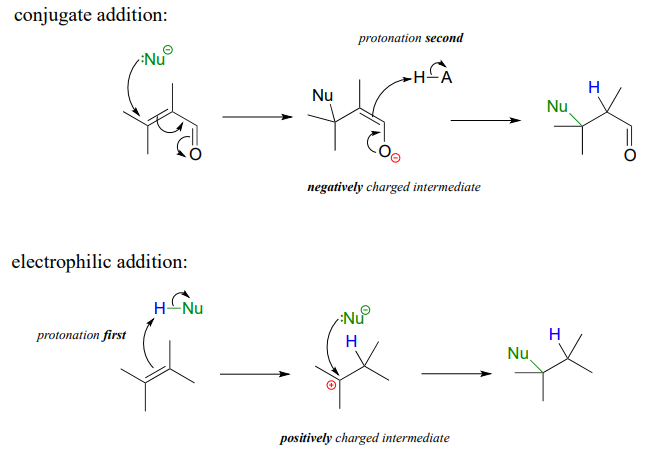
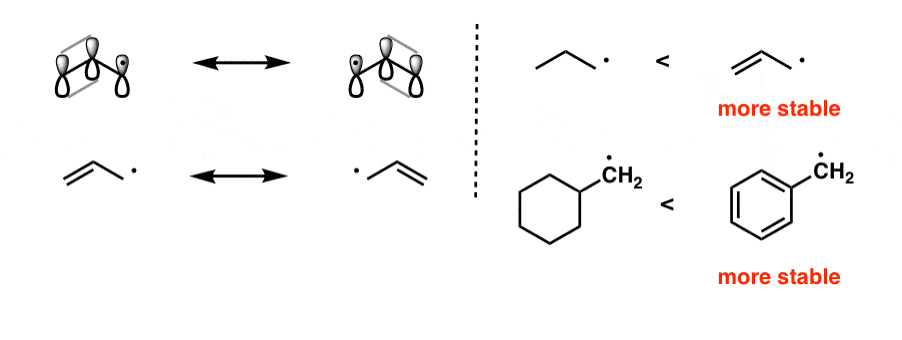
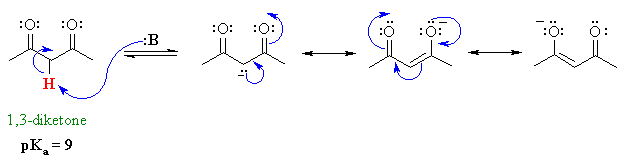
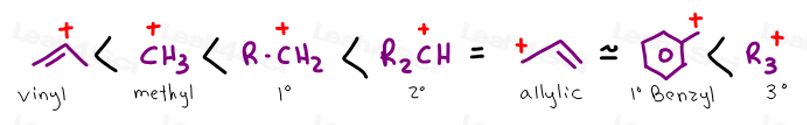
Vinylic carbon vs allylic carbon. It provides plenty of examples including allylic and vinyli. This organic chemistry video tutorial explains how to determine which carbocation is most stable. Allyl form a stable carbocation because of the electron delocalization whereas vinylic carbocations are unstable as they lack p character. Key difference allylic vs vinylic carbons functional groups are very important in understanding the different physical and chemical properties of organic molecules the terms allylic and vinyl carbons indicate whether the carbon atom is bonded directly or indirectly to a double bond in a molecule.
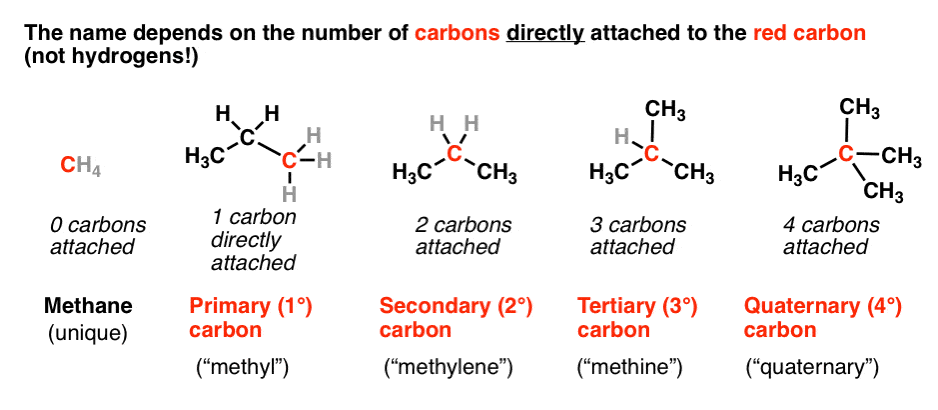
Allyl group holds three carbon atoms and five hydrogen atoms on the other hand vinyl group has two carbon atoms and three hydrogen atoms. The allylic carbon atom is more reactive than normal. Allyl indicates a functional group with structural formula h 2 c ch ch 2 r where r is the rest of the molecule it consists of methylene bridge ch 2 in between the vinyl group ch ch 2 and the rest of the molecule therefore allyl group contains sp 2 hybridized vinyl carbon atoms and sp 3 hybridized allyl carbon atom. The double bonded carbon atoms can be classified as vinylic and allylic carbon atoms.