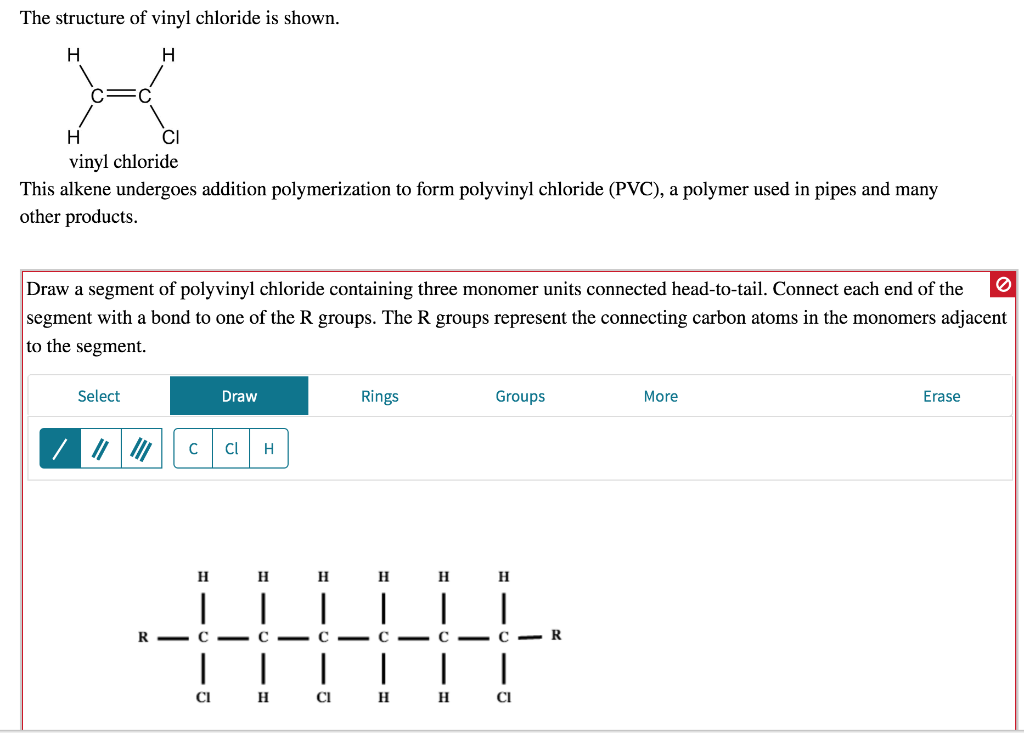
Vinyl Chloride Monomer Structure
Vinyl acetate is used to make other industrial chemicals.
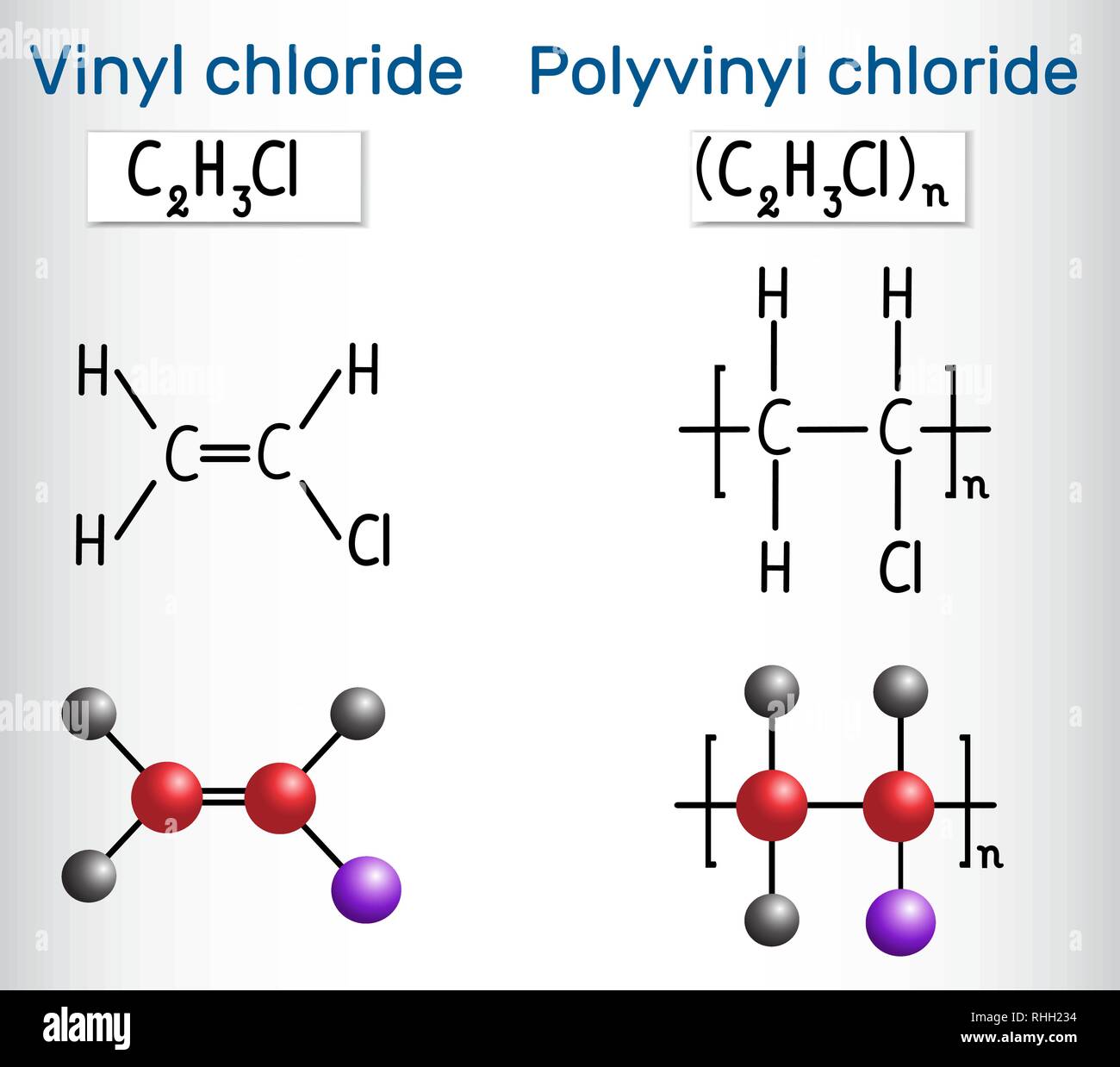
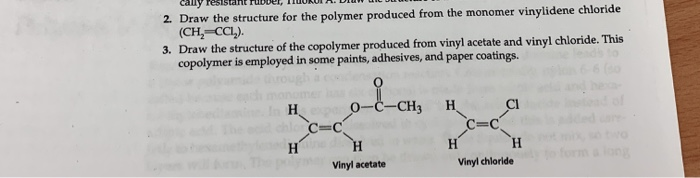
Vinyl chloride monomer structure. Specifically workers in polymerization section of a b f. Pvc is used in the manufacture of numerous products including packaging films and water pipes. Goodrich plant near louisville kentucky were diagnosed with liver angiosarcoma also known as hemangiosarcoma a. Vinyl chloride is an organohalogen compound that has important industrial applications.
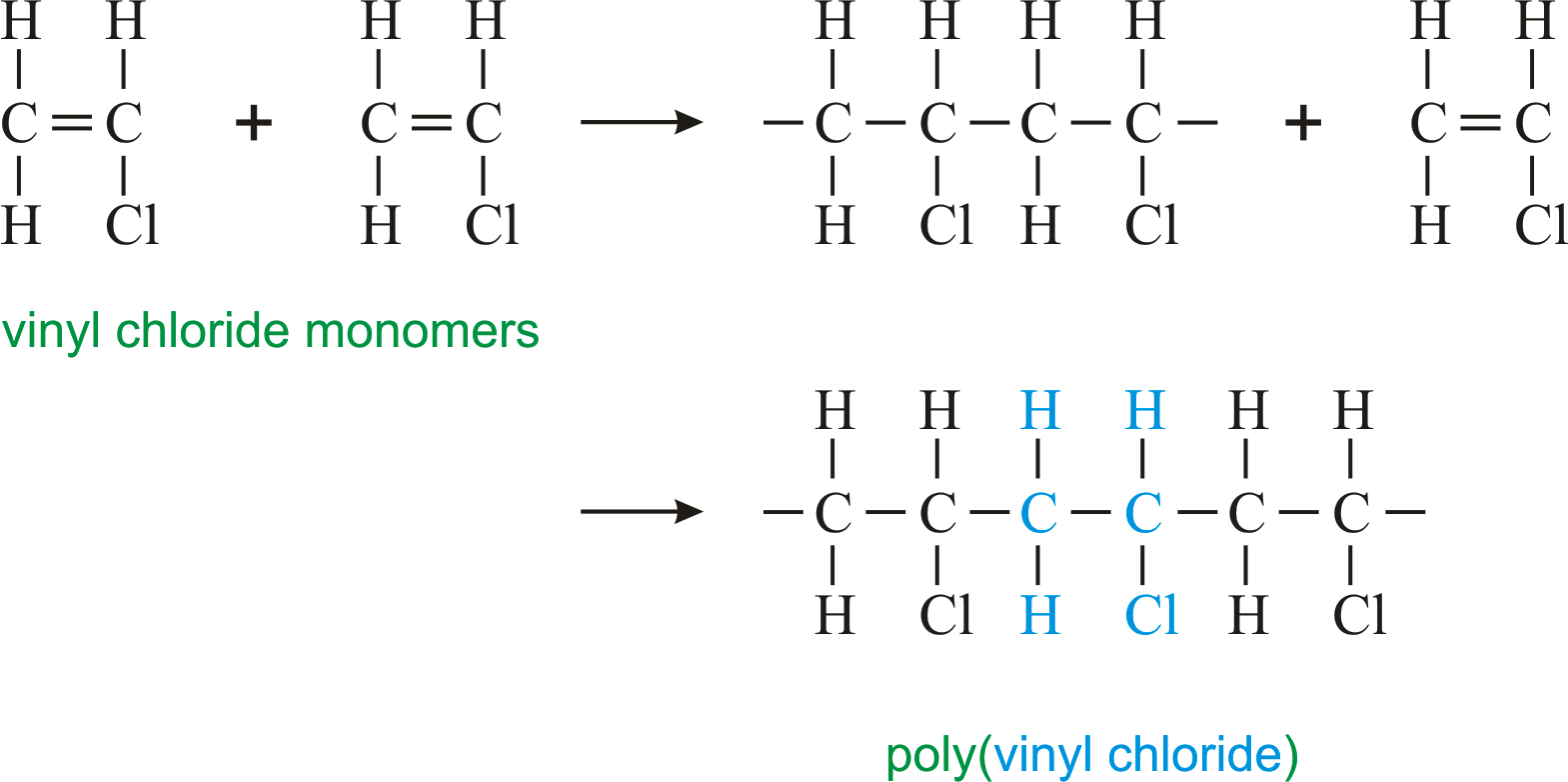
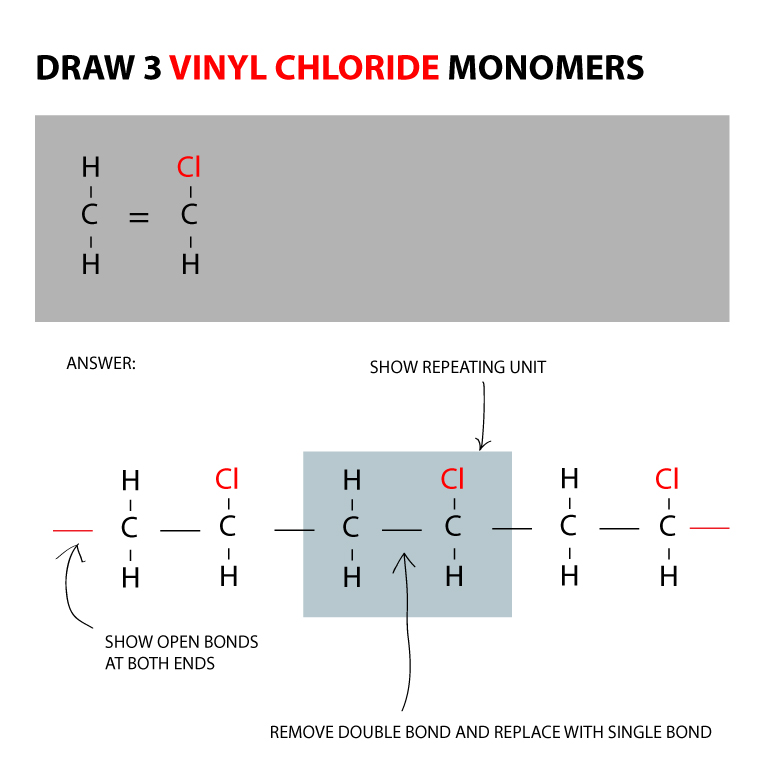
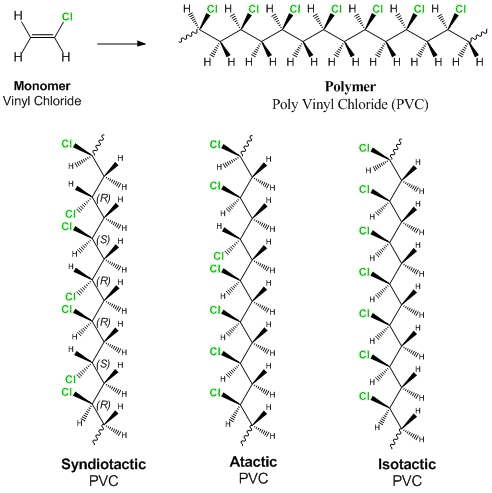
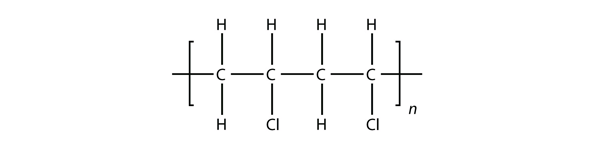
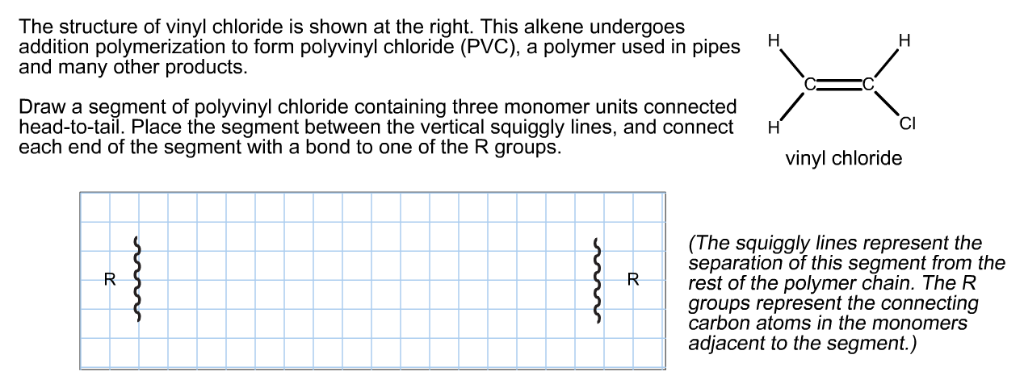
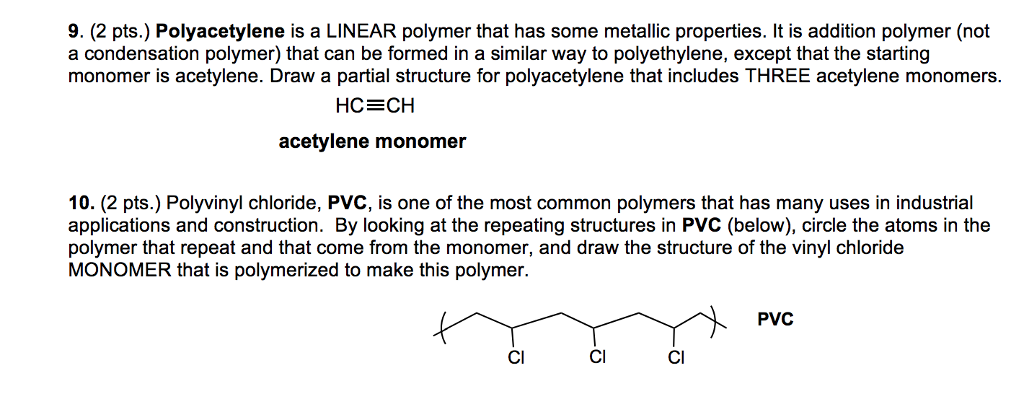
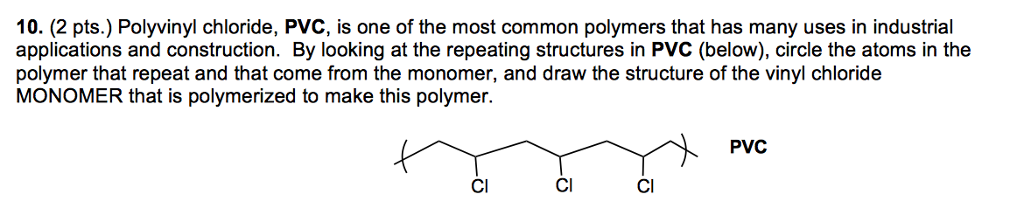
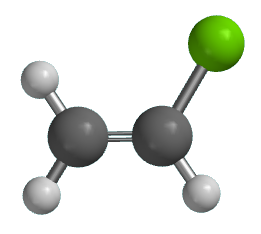
If generated an inchi string will also be generated and made available for searching. It is a carcinogenic gas that must be handled with special protective procedures. Vinyl chloride is primarily used to make polyvinyl chloride to manufacture plastics. When treated with certain catalysts vinyl chloride monomers undergo polymerization and form the larger compound known as polyvinyl chloride or pvc.
Polyvinyl chloride is produced in an addition polymerisation reaction from chloroethene vinyl chloride monomers. The molecular structure is based on structures generated from information available in echa s databases. The monomer chloroethene or vinyl chloride has a boiling point of 14 o c 259k so it is a gas at room temperature and pressure. It is very flammable and may be ignited by heat sparks or flames.
Vinyl chloride is a chlorinated hydrocarbon occurring as a colorless highly flammable gas with a mild sweet odor that may emit toxic fumes of carbon dioxide carbon monoxide hydrogen chloride and phosgene when heated to decomposition. Vinyl chloride is an organochloride with the formula h 2 c chcl that is also called vinyl chloride monomer vcm or chloroethene this colorless compound is an important industrial chemical chiefly used to produce the polymer polyvinyl chloride pvc. In the early 1970s the carcinogenicity of vinyl chloride usually called vinyl chloride monomer or vcm was linked to cancers in workers in the polyvinyl chloride industry. Vinyl acetate is an industrial chemical that is produced in large amounts in the united states.
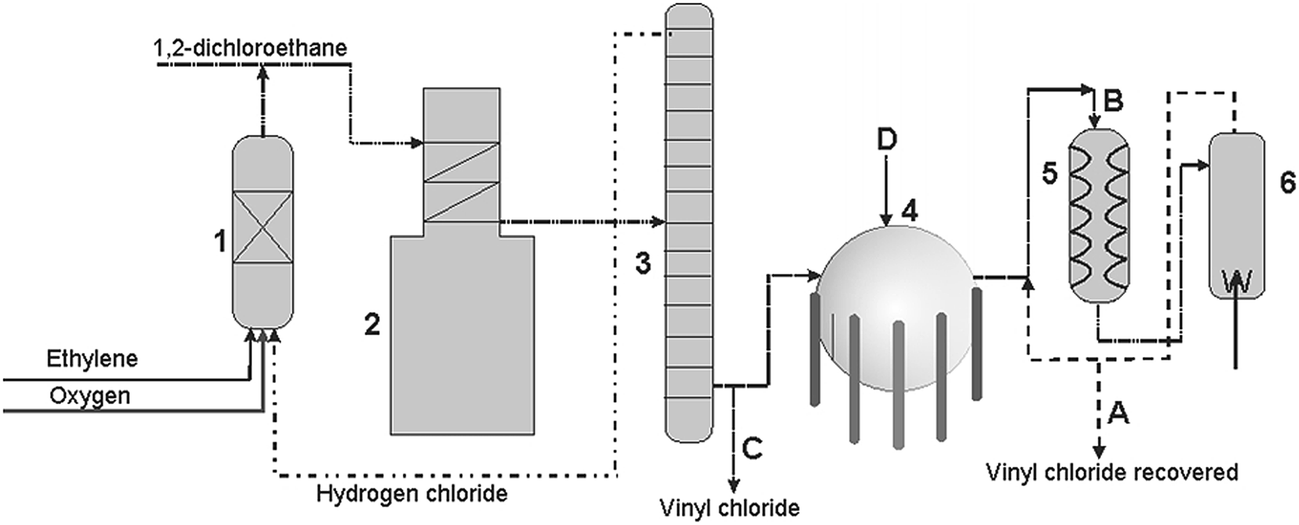
The balanced process is based on ethylene and the carbide process is based on acetylene. Under pressure the gaseous chloroethene molecules are forced closer together to form a liquid. Vinyl chloride monomer vcm is prepared commercially in two processes based on different two carbon hydrocarbons. Pvc is used to make a variety of plastic products including pipes wire and cable coatings and packaging materials.
Vinyl chloride ch 2 chcl is most often obtained by reacting ethylene with oxygen and hydrogen chloride over a copper catalyst. This information is only displayed if the substance is well defined its identity is not claimed confidential and there is. Vinyl chloride is used primarily to make polyvinyl chloride pvc. Vinyl chloride is also produced as a combustion product in tobacco smoke.