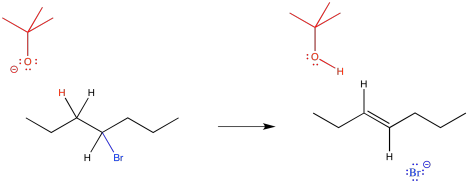
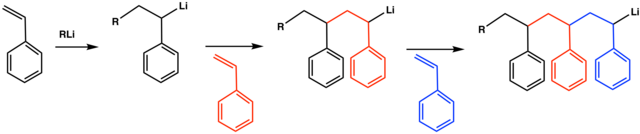
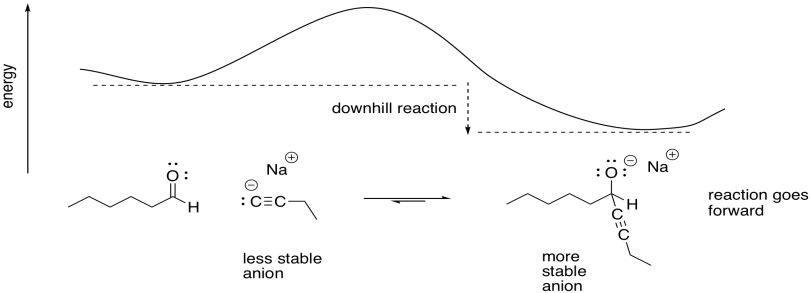
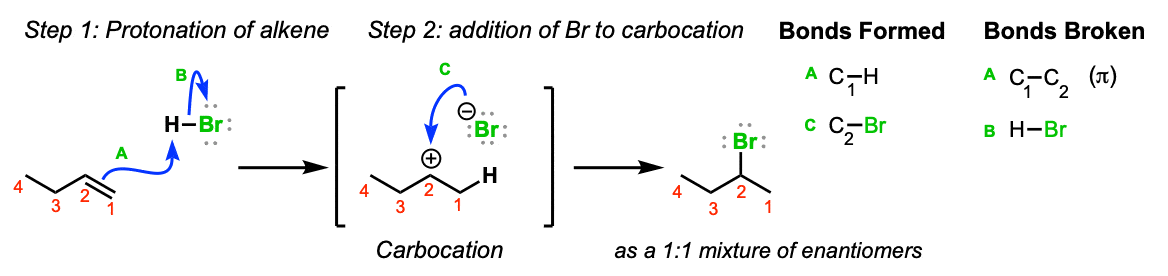
Vinyl Anion Stability
The decrease in the cco angle from 120to 113 0e kcal mole 24 8 3 3 2 1 no.
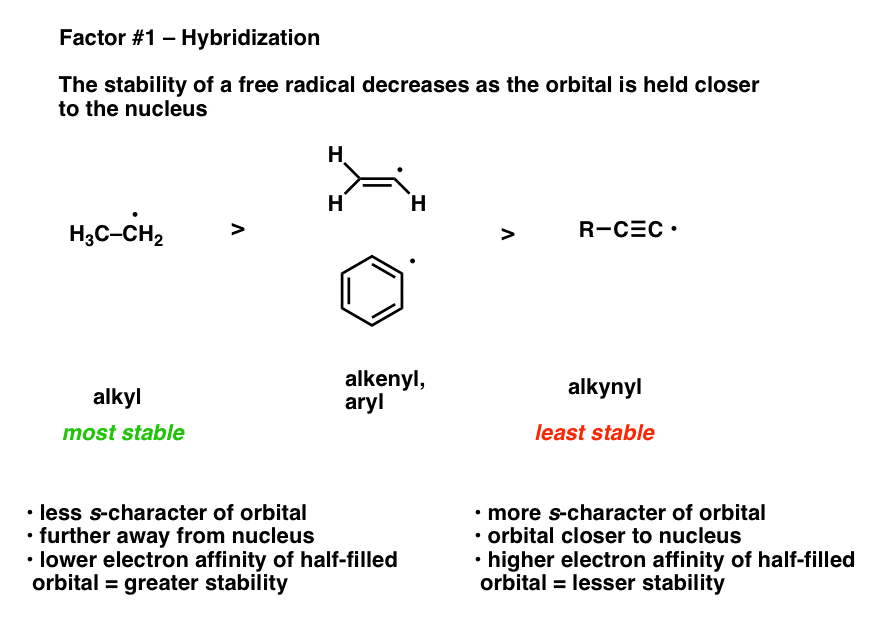
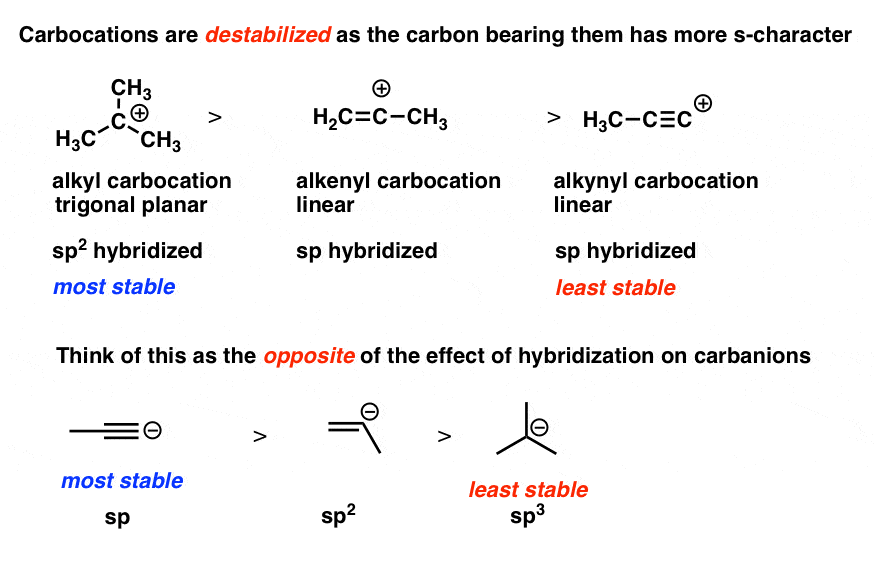
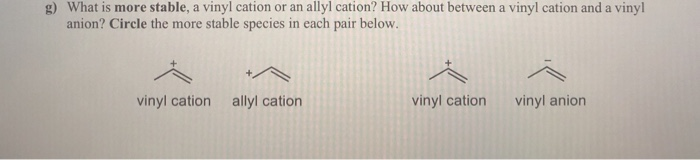
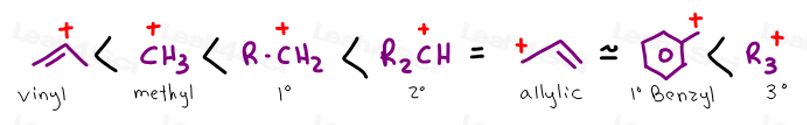
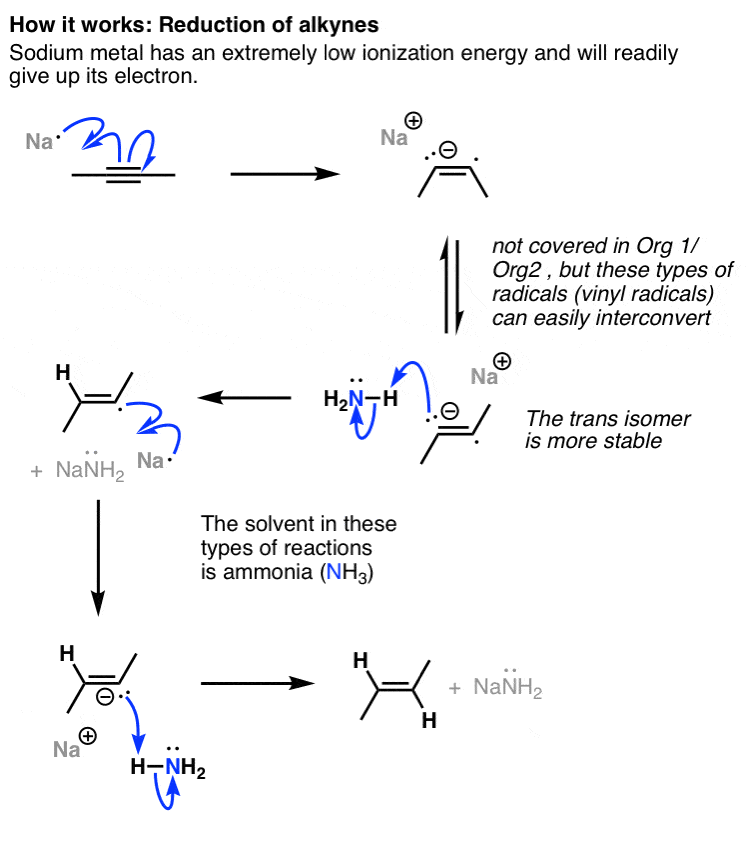
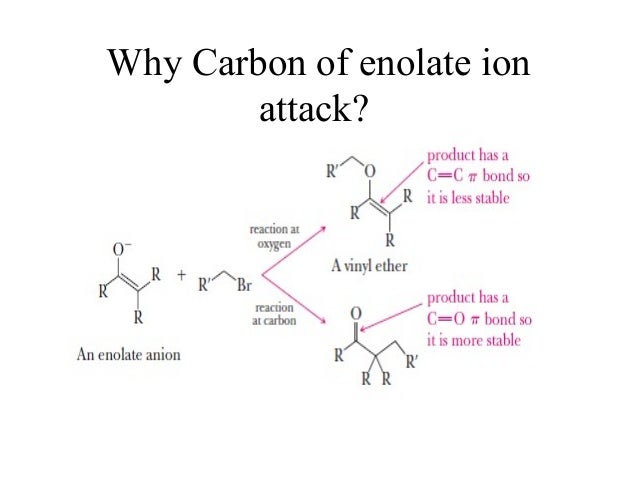
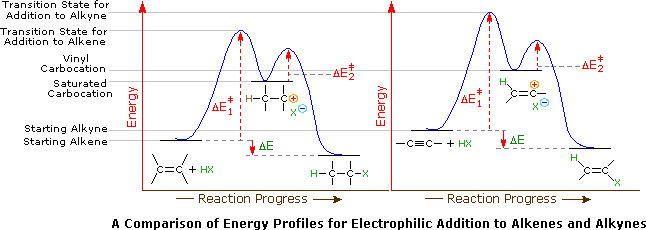
Vinyl anion stability. The vinyl cation is a carbocation with the positive charge on an alkene carbon. The value under de indicates the stability of the allyl anion compared to the partner vinyl anion. I would have thought that the carbanion would be bent with a barrier to inversion just like an imine. Class structure stability pattern carbocations c allylic 3º 2º 1º methyl alkenyl vinyl aryl electron poor electrophilic acidic carbon radicals c allylic 3º 2º 1º methyl alkenyl vinyl aryl electron poor.
Thus it is very important to know their stability patterns. That may be true for a vinyl cation but is it also true for the vinyl carbanion. This specially designed ion channel leads to. The lone pair would be more stable in an sp2 orbital rather than a p orbital.
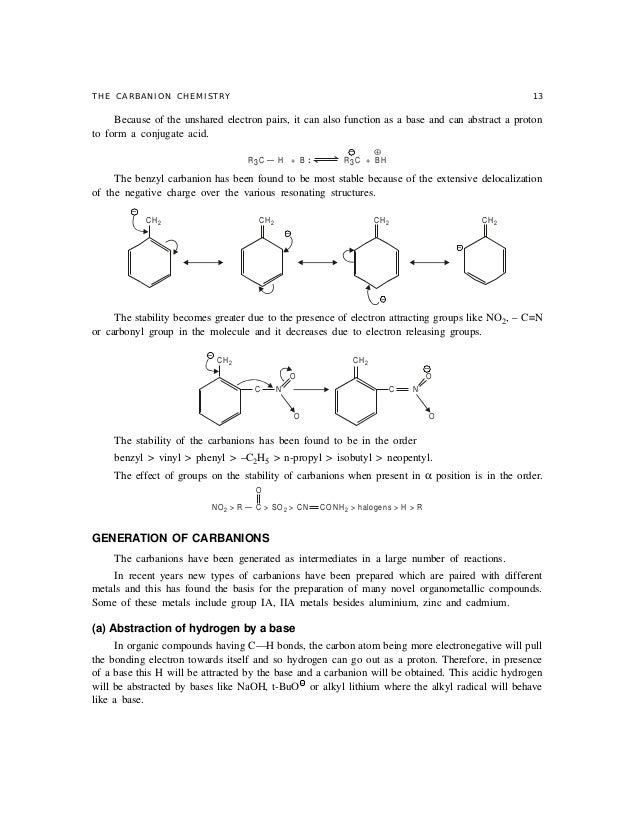
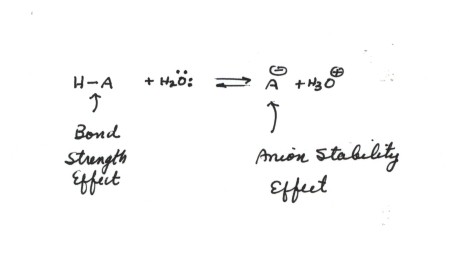
Of 0 06 a over the neutral 57 and indicates a repulsive interaction between the localized anion orbital and the lone pair orbital on the oxygen. A carbanion is an anion in which carbon is trivalent forms three bonds and bears a formal negative charge in at least one significant resonance form. An electron microscope clearly reveals a highly efficient ion channel network which is constructed with a small amount of cation exchange groups. A high performance anion exchange membrane aem is critical for the development of alkaline fuel cell.
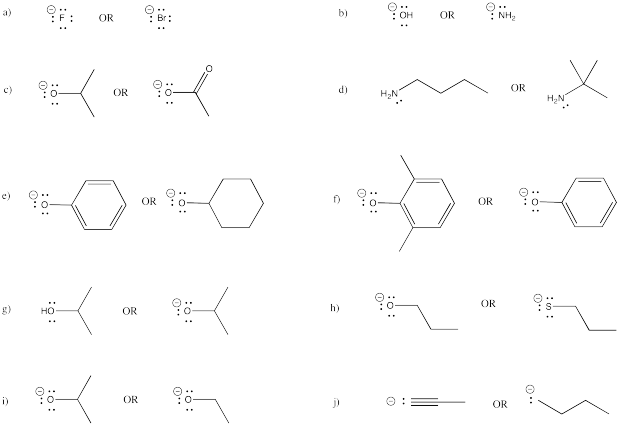
The relevant anions as shown in the figure are the ethynide anion in which carbon is sp hybridized the vinyl anion trigonally or sp 2 hybridized and the ethyl anion tetrahedrally or sp 3 hybridized the former is the most stable and the last named one least stable so that the rule of controlling anion stability is obeyed. Absent π delocalization carbanions assume a trigonal pyramidal bent or linear geometry when the carbanionic carbon is bound to three e g methyl anion two e g phenyl anion or one e g acetylide anion substituents respectively. In this work aems with an interpenetrating polymer network ipn are synthesized. Conclusion the configurational stability of a vinyl anion can be understood from the relative instability of intermediate 7 in its inversion process whereas the corresponding intermediate in the inversion of a vinyl radical 10 is relatively more stable leading to a lower energy barrier to inversion.